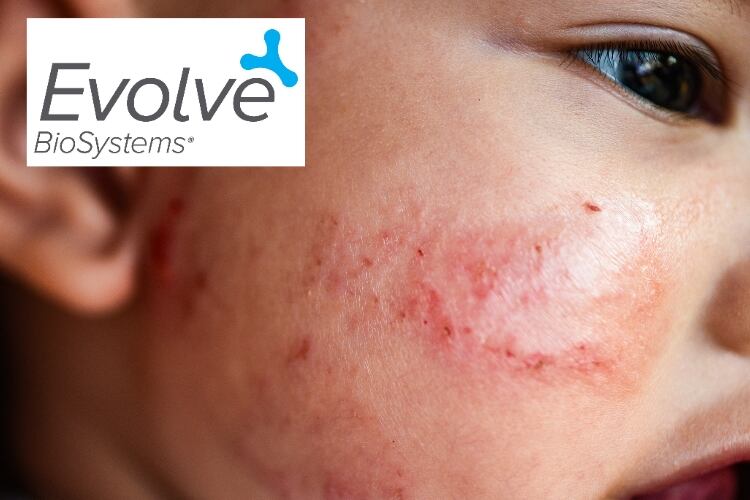
The study aims to investigate whether infant probiotic Evivo can reduce development of early childhood atopic dermatitis, which is implicated in asthma, allergic rhinitis and food allergies, and affects up to 20% of children.
The study will assess the effects of introducing a specific gut bacterium, Bifidobacterium longum subspecies infantis (activated B. infantis EVC001, marketed as Evivo) in inhibiting the onset of atopic dermatitis, the most prevalent pediatric skin disorder, in the first year of life.
The agreement was facilitated by Johnson & Johnson Innovation LLC.
"Atopic dermatitis is not just a debilitating condition for millions of children, but is also a gateway condition associated with numerous chronic conditions that carry a lifelong physical, emotional and financial burden," said David Kyle, PhD, chairman of the board and chief scientific officer, Evolve BioSystems, Inc.
"This study looks to demonstrate whether the introduction of B. infantis EVC001 to the infant gut microbiome will deflect the development of atopic dermatitis and establish a path to lifelong health."
Atopic dermatitis is an immunological disease condition that affects up to one in five children, with 60% of cases presenting by age one year.
Atopic dermatitis has been implicated in predisposing children to a range of pediatric health conditions ranging from food allergies to asthma. These individuals also have an increased risk of other conditions associated with inflammation, such as inflammatory bowel disease and rheumatoid arthritis, and are at increased risk of attention deficit/hyperactivity disorder and depression.
B. infantis EVC001 transforms Human Milk Oligosaccharides (HMOs) into quickly available energy for the intestinal cells. This lowers the gut pH, reducing potential pathogens by 80%. The loss of B. infantis due to C-section delivery and antibiotic use is linked to higher risk for atopic dermatitis, as well as a six-fold higher incidence of allergies and of type 1 diabetes in children.
The annual cost burden of atopic dermatitis in the US exceeds $5bn.
Study details
The study, a proof-of-concept, randomized, double-blind, placebo-controlled, two-arm parallel study, will be conducted in collaboration with Janssen. Investigators will enroll infants who are less than 15 days old, with a genetic predisposition to allergic conditions, and who are breastfed, with maternal intent to maintain breastfeeding through three months of age.
Study participants will be given either daily single doses of a supplement with B. infantis (EVC001) or a placebo for a 12-week period, and then monitored for signs of atopic dermatitis, skin rash, diaper rash and itch over the next two years.
Evolve BioSystems, Inc. is a privately held microbiome company dedicated to researching solutions to establish, restore, and maintain a healthy gut microbiome. Evolve is a portfolio company of the Bill & Melinda Gates Foundation and Horizons Ventures, the venture division of the Li Ka Shing Foundation.
Evolve is a spin-off from the Foods for Health Institute (FFHI) at the University of California, Davis.
The legal entity to the agreement with Evolve is Johnson & Johnson Consumer, Inc.